Alfred Schinkel
Pharmacology of anticancer drugs
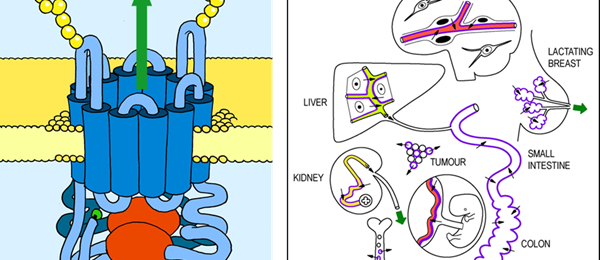
Anticancer drugs can be effective, but also highly toxic to patients. Moreover, tumors can develop resistance to anticancer drugs. We are trying to understand the mechanisms by which the body deals with anticancer drugs, and protects itself from their toxicity, as well as how tumors develop drug resistance.
Both processes turn out to be closely related. We aim to obtain insights into these processes using in vivo mouse models, helping us to develop better anticancer drugs and treatment approaches, optimize drug exposure, and minimize the risks of toxicity and development of drug resistance.