Study on actin filament disassembly published in Cell
The actin cytoskeleton constitutes a versatile network of filaments that controls the movement and morphogenesis of all eukaryotic cells. To rapidly change cell shape, these actin filament networks need to disassemble within seconds. However, the molecular mechanisms underlying this process had remained elusive for a long time.
In this new study that wraps up Wout’s postdoctoral work, we uncovered how the three proteins coronin, cofilin and AIP1 work together to rapidly disassemble actin filaments.
Using cryo-electron microscopy (cryo-EM), we determined sixteen different states of the actin filament disassembly process. This revealed a concerted mechanism of protein rearrangements that involved several coordinated steps. Thus, we uncovered a ‘molecular choreography’.
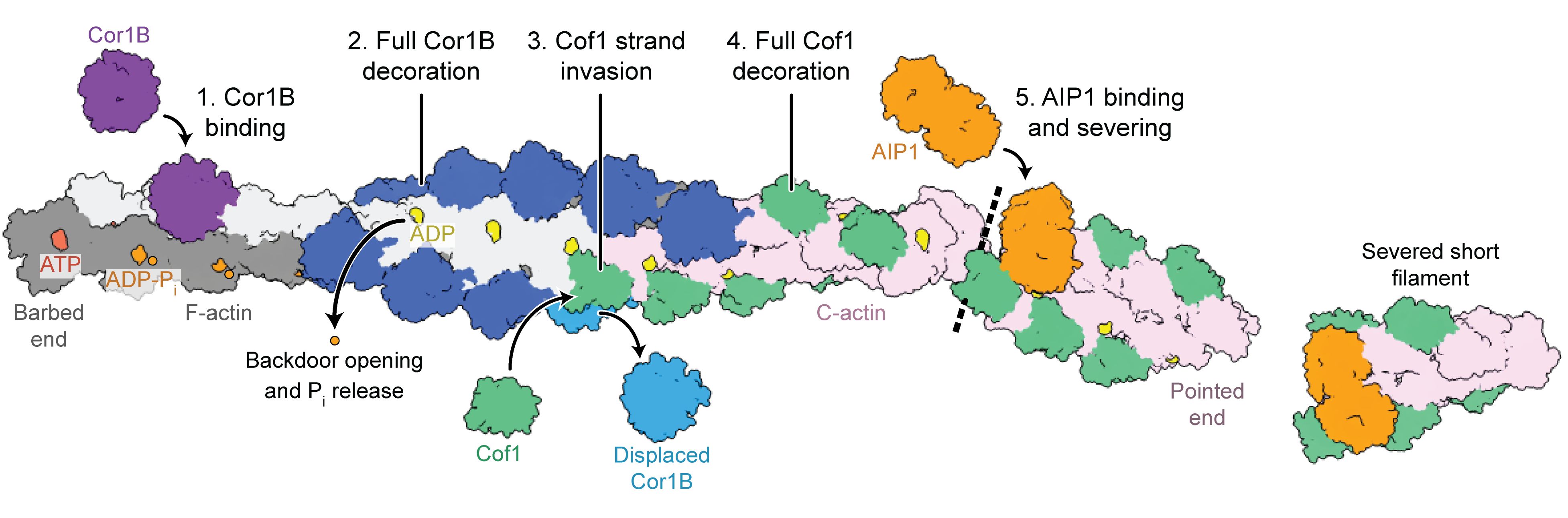
First, coronin (Cor1B, purple and blue) binds to the actin filament (grey and pink). This facilitates the binding of cofilin (Cof1, green) to the filament and leads to the release of coronin. Finally, AIP1 (orange) engages cofilin-bound actin to sever the filament, resulting in rapid disassembly. In other words, AIP1 breaks the filament and splits it into two shorter fragments.
Importantly, the dysregulation of this actin disassembly process is associated with a variety of diseases, ranging from cancer to myopathies. Our fundamental work now sheds light on the specific roles of the proteins coronin, cofilin and AIP1 – thereby paving the way for future therapeutic studies.
Interested to find out more? Read our manuscript.