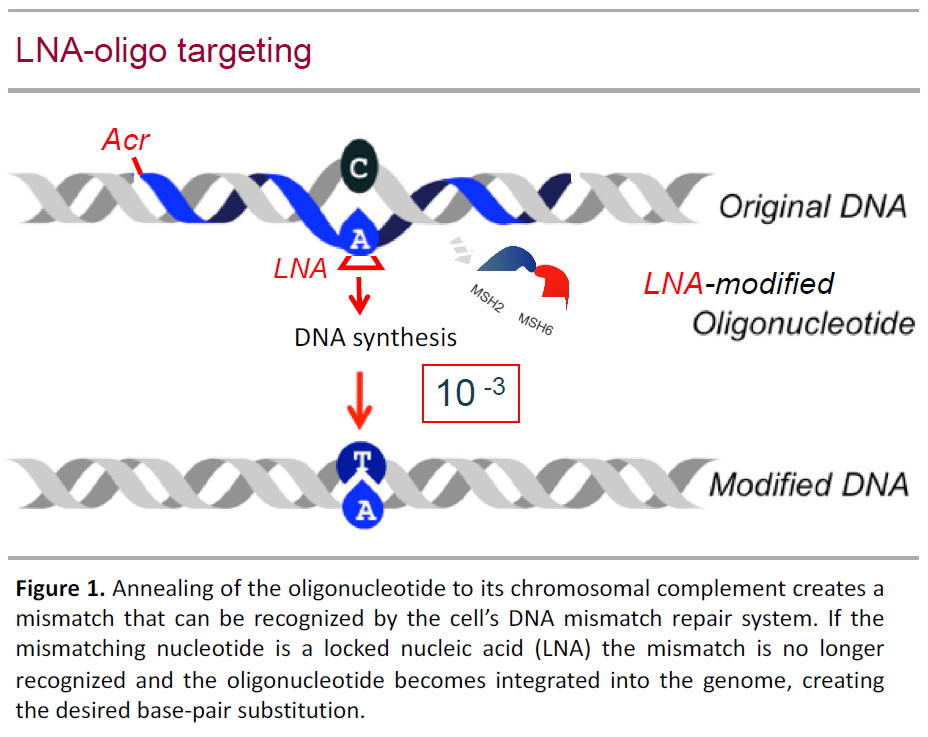
We use oligo targeting to study so-called ‘variants of uncertain (clinical) significance’ (VUS) of MMR genes that are found in suspected LS patients. Evidently deleterious germ-line defects in MMR genes (e.g., protein-deleting or -truncating) allow for the clinical diagnosis LS and carriers can be offered surveillance to reduce cancer risk. However, single codon variants are difficult to interpret complicating the counseling of carriers as long as it is uncertain whether the variant is disease-causing or innocuous. To study such ‘Variants of uncertain significance’ (VUS), we developed an oligo-targeting-based screen: “oligonucleotide-directed mutation screening” (ODMS) (Houlleberghs et al., PNAS 2016; PLoS Genet 2017; J Med Genet 2020). In our current protocol, we first design an appropriate LMO to introduce the VUS into ±0. 1% of human diploid cells that were made hemizygous for a MMR gene. When the VUS is deleterious, modified cells form colonies in MNNG-containing medium; when no colonies appear, all cells remained MMR proficient indicating the VUS is innocuous. We are constantly improving this protocol and are implementing ODMS in clinical practice as a routine diagnostic tool with the help of a nationwide Dutch-Cancer-Society-sponsored consortium, termed INVUSE (“investigating variants of uncertain significance for use in clinical practice”).
ssODN-mediated gene modification can also occur outside the context of DNA replication when stimulated by a CRISPR/Cas9-induced DNA break. Strikingly, DNA MMR impacts ssODN-directed gene modification without and with nuclease activity differently: while suppressing replication-coupled oligo targeting, break-assisted gene modification necessitates MMR to promote break-distal nucleotide substitution instructed by the 3’-half of the ssODN. This finding implies templated break repair rather than oligonucleotide integration to underlie CRISPR/Cas9-mediated nucleotide substitution (Harmsen et al., NAR 2018).
We corrected a disruptive mutation in the Fanconi anemia (FA) gene Fancf using CRISPR/Cas9 and a 120-nt ssODN template in mouse ESCs and fibroblasts. Although the frequency was low (3-6%), FA-corrected ESCs rapidly overgrew non-corrected cells, which even allowed recovery of very rare templated gene editing events obtained by using Cas9D10A nickase. Notably, unlike wild-type Cas9, nickase activity resulted in mono-allelic gene editing without undesired mutagenesis (Van de Vrugt et al., Sci Rep 2019).
We generated a novel mouse model in which, similar to LS patients, MSH2-defective crypts arise amidst an excess of MMR-proficient crypts (ratio 1:20) (Wojciechowicz et al., Gastroenterology 2014). Half of these animals spontaneously developed MSH2-deficient intestinal tumors after ±1.5 year. Exposure of “Msh2-Lynch” mice to the methylating agent temozolomide caused 5-fold expansion of MSH2-deficient crypts and dramatically accelerated intestinal tumorigenesis, reducing the latency to ± 4 months in all animals. Exposure to methylating agents is clearly a risk factor for tumor development in LS patients. Remarkably, tumor incidence was strongly reduced when animals were sanitized and housed under SPF conditions. We are currently investigating which environmental factors impact tumor development. Ultimately this work may yield prophylactic strategies to reduce cancer risk in LS patients.
Loss of G1/S control is frequently seen in cancer. Genetic ablation of the retinoblastoma (Rb) proteins pRB, p107 and p130 disrupts G1/S control, causing unscheduled S-phase entry, but not indefinite proliferation. Apparently, additional events are needed for oncogenic transformation. To identify these, we study the cell cycle behavior of Rb-protein-defective cells.
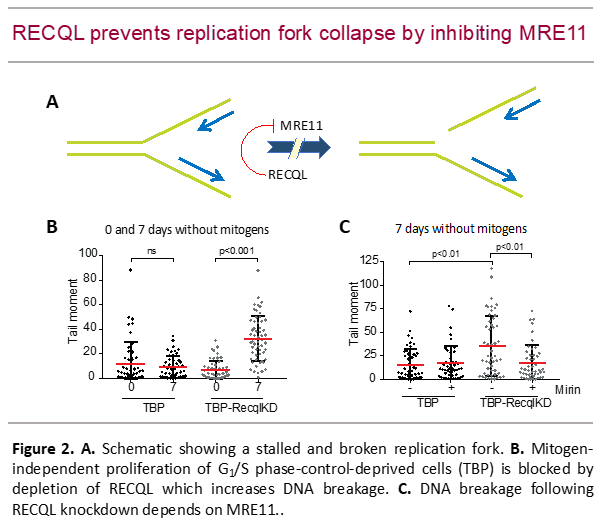
Unscheduled S-phase entry, e.g., in the absence of mitogenic stimuli, of G1/S-checkpoint-defective cells causes replication stress, revealed by slow fork progression, low origin firing, DNA breakage and proliferative arrest (Foijer et al., Cancer Cell 2005; Van Harn et al., Genes Dev 2010). Strikingly, mitogen-independent proliferation was made possible by disruption of the Tp53/p21CIP1 axis, not only by attenuating the DNA damage response, but rather by restoring origin firing and reducing DNA breakage (Benedict et al., Elife 2018). However, replication speed remained low, indicative for sustained replication stress. We reasoned that proliferation under these conditions was dependent on cellular pathways that mitigate the deleterious consequences of replication stress. Indeed, we found that replication forks frequently collapse but are rapidly reinstalled by RAD51-dependent double-strand break repair that required WAPL-dependent removal of cohesion rings (Benedict et al., Dev Cell 2020). Furthermore, we found cohesion loss in many cancer cell lines, suggesting that active cohesion removal from newly synthesized sister chromatids is a widely-used strategy cancer cells use to handle oncogene-induced DNA replication stress.
Subsequent genetic dropout screens revealed additional vulnerabilities of cancer cells suffering from replication stress. that may provide novel leads towards anti-proliferative therapy. E.g., we found a requirement for the helicase RECQL that prevents replication fork collapse by inhibiting MRE11 nuclease activity (Benedict et al., Life Sci Alliance 2020; Figure 2).