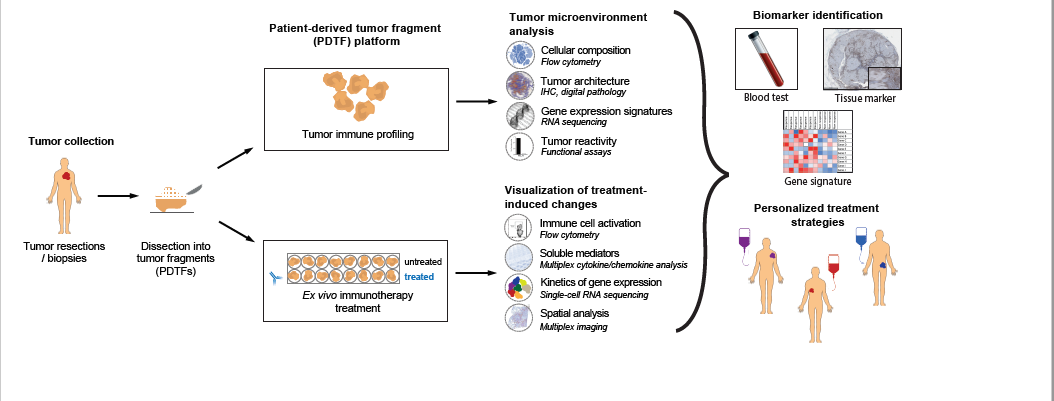
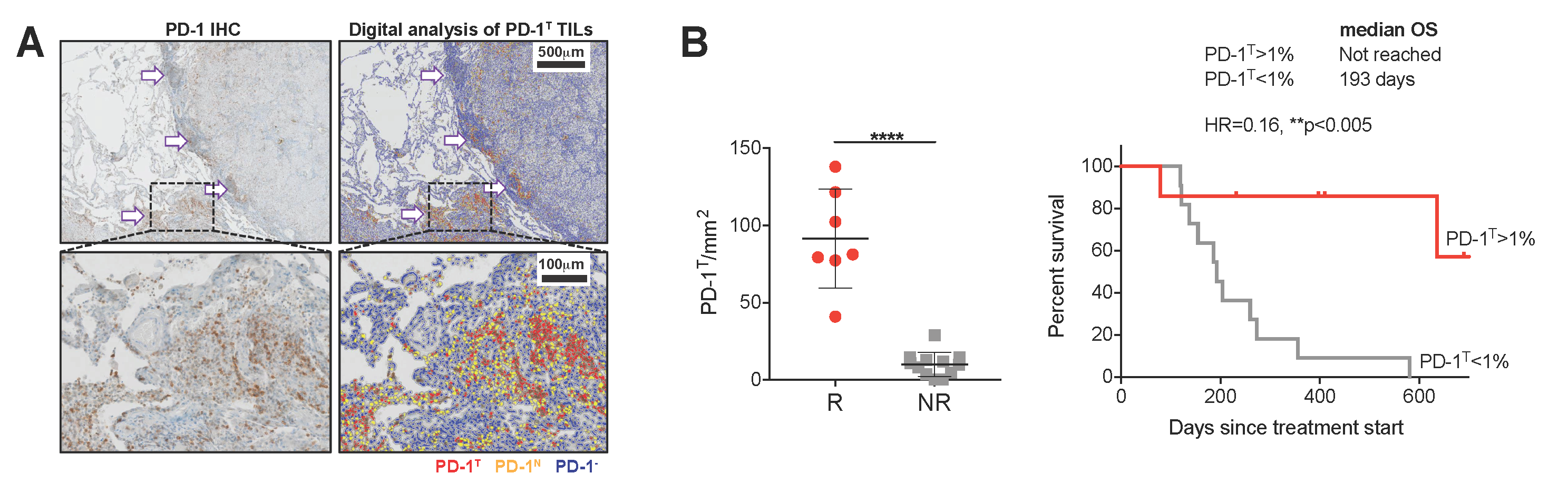
Precision immunotherapy in human cancer
Over the last years, cancer immunotherapy, i.e. immune checkpoint inhibition (ICI), has truly transformed the therapeutic field in cancer and is now approved for the treatment of many tumor types. Nevertheless, the number of meaningful clinical responses is still rather low. Current available biomarkers do not allow to unambiguously identify patients who do or do not benefit from the therapy, exposing them to ineffective treatment and unnecessary toxicities. In order to improve patient selection and develop more personalized immunotherapy strategies, a better fundamental understanding is needed of how human cancers immunologically respond to treatment and how such responses relate to the immunological makeup of a tumor.