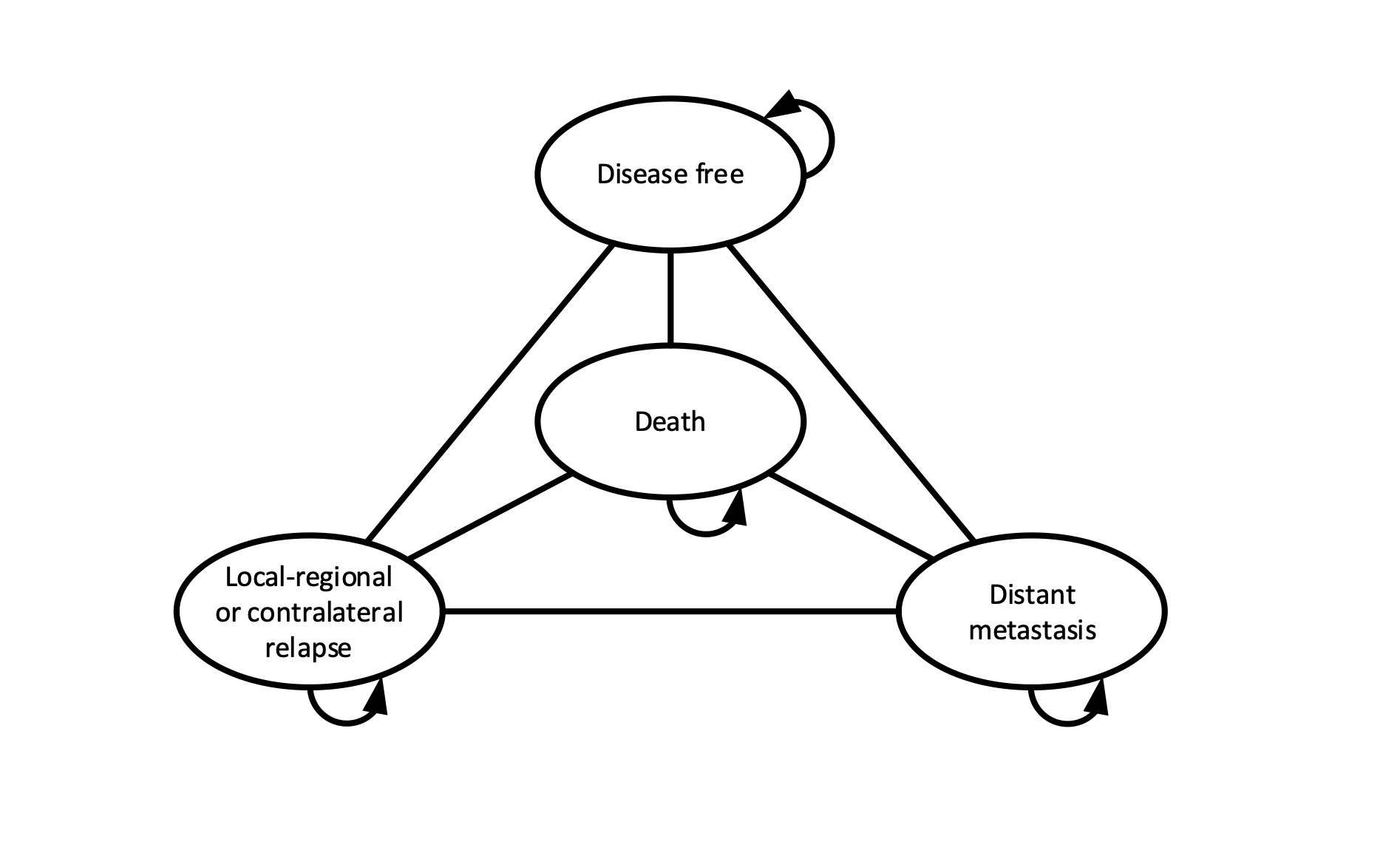
In Cost-effectiveness models, we can compare the expected costs and outcomes for new or existing interventions.
A CEA can be performed based on a phase III clinical trial, where the goal is to determine the cost-effectiveness of a new intervention.
An early CEA can be performed in an earlier stage of development, in this case the goal is to steer towards cost-effectiveness, and support further R&D.
Patients & professionals preferences
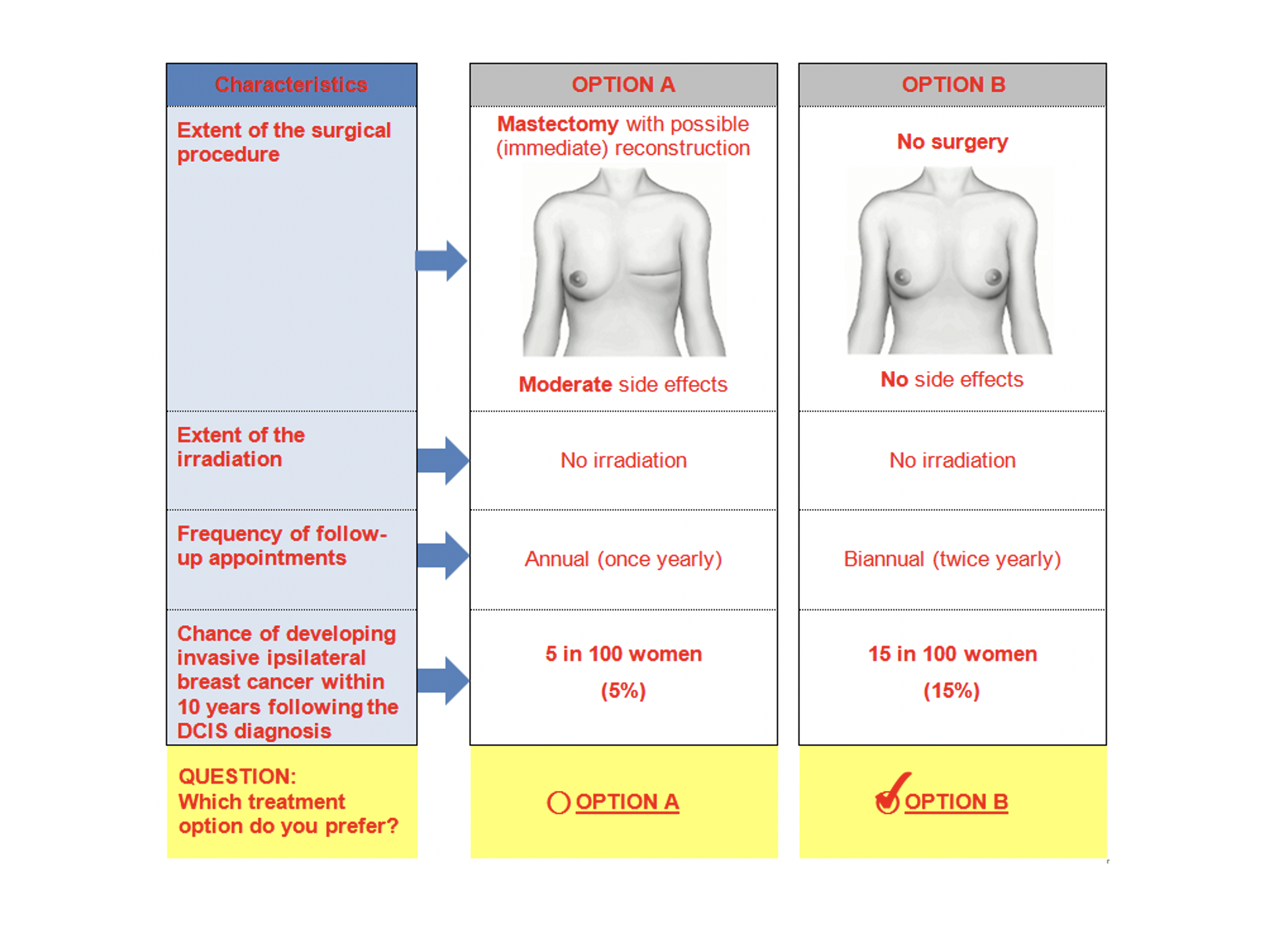
Discrete Choice Experiments (DCE)
The goal of DCE is to understand how patients and other stakeholders value various aspects of an intervention in healthcare.
Scenario drafting
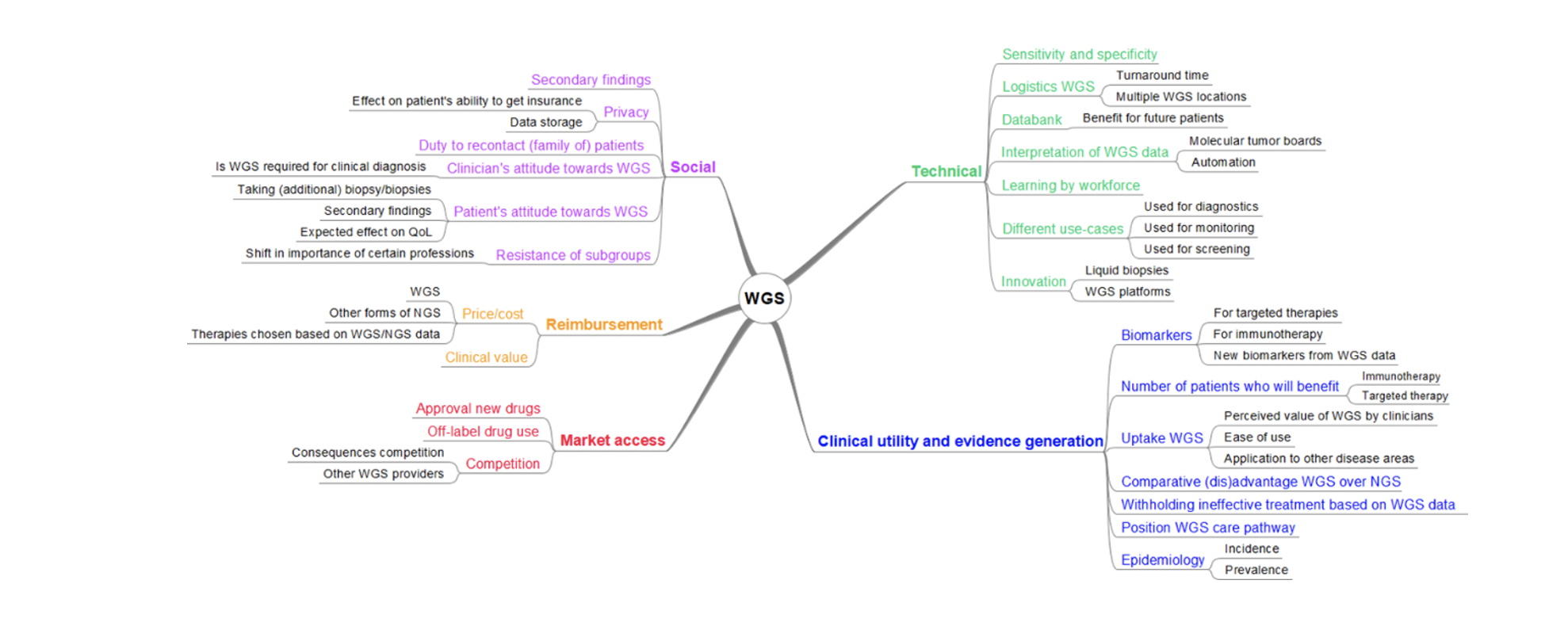
Scenario drafting
Using scenario drafting reflecting different future pathways, the HTA can support the adoption process in guiding development and reimbursement decisions, stepwise reducing the uncertainty in early stages of development.
Scenario analysis will consider the developments of a new technology, consisting of 1) Identifying (dynamic) aspects having impact on adoption, 2) brainstorm on possible scenarios (informal interviews with experts), 3) scenario drafting, 4) validation of scenarios, and 5) quantification into parameters for cost-effectiveness modeling. The scenarios can consist of aspects that can be patient-related, ethical, legal, organizational, and/or economical. The results of the scenarios will be quantitatively incorporated in cost-effectiveness models to be able to steer towards the most cost-effective solution.
Coverage with Evidence Development (CED) programs

Coverage with Evidence Development (CED)
Voorwaardelijk toelatings traject/ Veelbelovende zorg
CED programs (or in Dutch: veelbelovende zorg) are specifically for highly promising new technologies or treatments which are conditionally reimbursed while the phase III trial is ongoing.
Our team supports groups in the NKI-AVL for submission and coordination of the program, in collaboration with ZonMW and the ZorgInstituut