Cancer forms a huge global challenge, causing significant morbidity, mortality, and economic burden while compromising quality of life. The crux of the issue lies in the fact that most cancers are detected only when symptoms manifest, often at a late stage when curative options are limited and costs, both economic and in terms of health impact, soar. Recognizing this urgency, the World Health Organization (WHO) strongly advocates for cancer screening. Even in countries with robust healthcare systems, many cases are diagnosed late, underscoring the critical need for early detection.
Currently, cancer screening programs have been implemented in only a limited number of countries, and for a limited number of most common tumor types, mainly cervical, breast and colorectal cancer, and to a lesser extent for prostate and lung cancer. Consequently, early detection of most types of cancers is still far behind the horizon.
The concept of ctDNA-based MCED tests offers a promising avenue for advancing population-based cancer screening. Yet, significant hurdles persist. Proof of concept on how to organize multi-cancer logistics screening in a programmatic setting is lacking. There is limited, clinical validation of suitable candidate MCED tests in target populations. Also, several ethical, legal and societal implications (ELSI) so far hardly have been addressed, one of which is the risk of lead time bias.
In addition to having a better test, improved identification of an individual’s risk of cancer (the pre-test likelihood) can increase the predictive value of cancer screening tests, optimizing the benefit/harm ratio of cancer screening e.g. by personalizing age limits, cut-offs or screening intervals. Improving individual risk assessment through hereditary polygenic risk scores (PRS) and exposome risk scores (ERS) holds potential for optimizing the effectiveness of screening tests. To this end, hereditary polygenic risk scores (PRS) of cancer, as well as exposome risk scores (ERS, including internal and external measurements of lifestyle, environmental, and social factors) hold potential, but await further evaluation. Since germline DNA is present in the same blood sample as used for measuring ctDNA, ctDNA based screening provides an extra opportunity here. However, the combined diagnostic value of PRS and ERS alongside multi-cancer screening tests requires further substantiation.
Cost-effectiveness is a major factor in political decisions on whether to implement screening, also for multi-cancer ctDNA-based screening. Currently any reference base for the performance of such tests in the Dutch setting is lacking.
The primary objective of the ESCALATION project is to generate reference data on multi-cancer ctDNA-based screening, coupled with PRS and ERS, representative for the Dutch setting. While the project aims to take an integrated approach, by no means it claims to be exhaustive. Rather the ESCALATION project aims to create a foundation to build on for further innovations of the highly organized Dutch cancer screening programs, by bringing together all important stakeholders in this innovation process chain, from academia and industry to public health parties and patient representatives.
Cancer early detection, and more specifically programmatic population-based screening is complex, which is also clear from the (updated) Wilson & Jungner criteria also supported by WHO1. Ideally, each of these criteria should be checked for every tumor, based on the highest level of evidence (systematic reviews, clinical trials). For some tumor types this has been done, for many others mainly assumptions exist. It is fair to say that also the ESCALATION project will not be able to address all of these in detail, but it is important to at least document their status explicitly. Most important assumptions are listed below. The ones in bold will be tested in the ESCALATION project.
-
- Detection and interception of cancer at an early or even pre-cancer stage reduces the cancer burden
- Better understanding of disease mechanisms (e.g. DNA alterations during cancer development) can lead to better biomarkers
- Generic disease mechanisms (e.g. DNA fragmentation) exist that allow for developing multi-cancer tests
- More tumor types than currently screened for could be detected early using a multi-cancer screening test
- An individual’s risk of cancer is determined by genetic and environmental/exposome factors
- The positive and negative predictive value of a screening test is determined both by test characteristics (sensitivity and specificity) and the pre-test likelihood (“risk”) of an individual for the disease screened for.
- Screening is the preferred term for cancer early detection efforts in the general population at average risk. “All screening programmes do harm; some do good as well, and, of these, some do more good than harm at reasonable cost”2. A key principle of cancer early detection research therefore is to optimize this benefit/harm ratio.
- Most effective cancer screening is achieved when organized in formal programs1.
- Screening programs (and the tests they use) should comply with the (updated) Wilson and Jungner criteria1.
- Cost effectiveness is a major factor in adoption/implementation of cancer screening programs.
- Early health technology assessment using mathematical modelling provides at an early stage of test development a good indication of the cost-effectiveness of the test under development.
- The impact of a cancer screening program is not only determined by the parameters of the test used, but also by its acceptance by the target screening population
- A blood-based test that measures generic features directly related to actual (pre)cancers provides the best opportunity towards a multi-cancer (or multi-cancer) screening test
The following specific research questions will be addressed in ESCALATION:
-
- Can ctDNA-based cancer screening logistics be implemented in a blood-bank setting?
- What is the accuracy for a multi-cancer ctDNA test across different tumor types in a (close to) target population?
- Do tangible risk factors like A) PRS and B) ERS provide added value?
- Is early Health Technology Assessment (HTA) of this multi-cancer screening approach positive?
- What ethical, legal, and social issues (ELSI) arise with this multi-cancer screening approach?
Ultimately, ESCALATION will provide a benchmark of the potential of ctDNA combined with polygenic and exposome risk scores in population-based multi-cancer screening.
To answer these research questions, we will collect a prospective large-scale (observational) cohort of samples among participants of the Dutch national blood-bank Sanquin. Within a nested case-control study, we will use the DELFI DNA fragmentation assay in combination with ERS and PRS for multi-cancer early detection.
Based on fundamental understanding of genome-wide DNA fragmentation mechanisms, and by applying artificial intelligence to large genomic data sets, a fragment analysis based circulating tumor DNA cancer early detection blood test has been developed. In addition, this new test is combined with tangible measures of genetic and environmental risk (i.e. polygenic and exposome risk scores), application of which in a screening setting is also novel. The proposed study is at a screening scale, representing a (near to) screening target population. Briefly, in ESCALATION, we aim to collect blood from ~125,000 blood-bank donors at screening age (50-75 years-old). By 1) linking to the National Cancer Registry, we expect to identify n=1,500 cases (i.e. diagnosis of cancer < 1 year). In a nested case-control study of n=6000, matched 1:3, we will measure ctDNA (i.e. DNA fragmentome patterns) as a potential multi-cancer screening test with or without additional risk stratification based on the PRS and ERS profile. In addition, we will 2) link to the Dutch national pathology archive (PALGA)3 in order to correlate ctDNA data to the incidence of premalignant lesions (e.g. colorectal polyps, epithelial dysplasias in several organs, etc.), as well as 3) to the RIVM screening database in order to correlate ctDNA data to the results of standard screening tests (i.e. mammography for breast cancer, HPV test for cervical cancer, and fecal immunochemical test for colorectal cancer). Moreover, we will repeat the linking to these three registries two and three years after blood collection to update the data. The data generated on ctDNA, PRS and ERS will be fed into HTA models to evaluate whether ctDNA-based multi-cancer screening could be clinically meaningful and acceptable from HTA perspective, and whether germline (PRS) and environmental (ERS) information have added value in predicting cancer risk. Moreover, ethical and social aspects will be evaluated.
The proposed project (Figure 1) is in several ways disruptive. First, we move from a cancer type specific screening approach to a multi-cancer approach. Second, next to introducing ctDNA as a new screening modality, we also add tangible estimates of genetic and environmental risk to the program that go substantially beyond the state of the art of only using sex and age as risk factors. Third, the set-up of recruiting participants from a blood-donation program may open new avenues towards potential future implementation, as donor blood nowadays already is tested for several disease entities, thus providing a rather straight forward avenue for scaling up to population-based blood-based cancer testing, without having to develop new logistics and infrastructure. Furthermore, explicit attention is being paid to early HTA and ELSI. Such a project can only be successfully executed by a multidisciplinary team of renowned leaders in the field of cancer early detection.
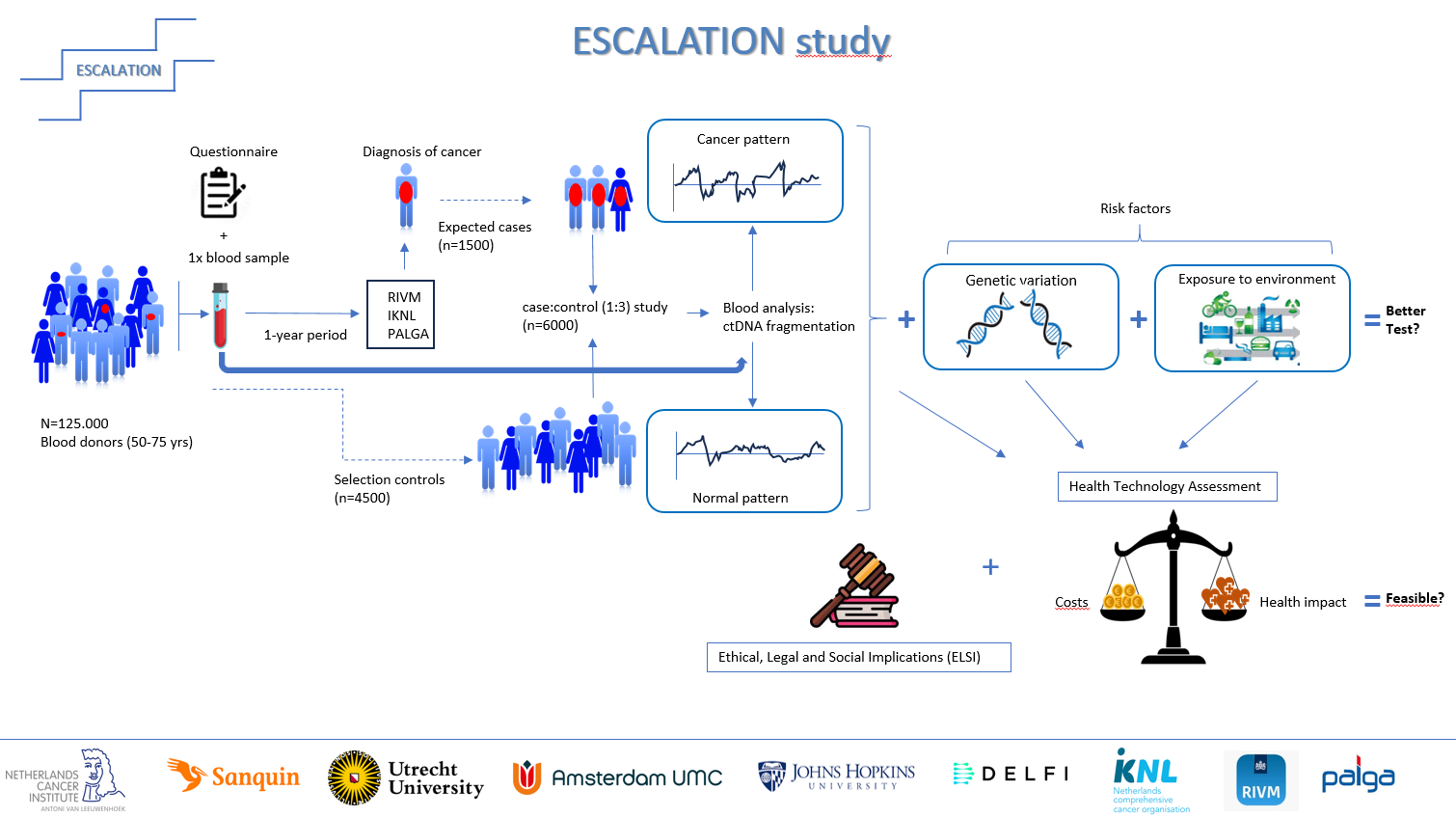
Figure 1. Schematic representation of the ESCALATION project, click to enlarge.